A chemical bond is the electrostatic force that unites two or more atoms. An atom is the smallest particle that retains all the chemical characteristics of an element. All substances, whether solid, liquid or gaseous, are composed of atoms. Atoms in turn are formed of even smaller particles: a nucleus of protons and neutrons, surrounded by orbiting electrons. Protons and electrons have an electrical charge. Protons have a positive charge and electrons have a negative one. Usually, the number of protons is equal to the number of electrons inside an atom. So their respective charges balance each other out, and the atom’s total charge is equal to zero.
Sometimes, however, in order to reach a more stable electric configuration, an atom captures one or more of another atom’s electrons. As a result the number of its electrons is superior to its protons, and the atom’s charge becomes negative.
This type of atom is known as an anion. Meanwhile, an atom that loses electrons has more protons than electrons, and its charge becomes positive. This kind of atom is called a cation. Since they have opposite charges, the anion and the cation have a strong reciprocal attraction: an ionic bond. But ionic bonds are only one of the various chemical bonds possible. Another kind of bond is formed when two atoms share one or more pairs of electrons. Each of the shared electrons is attracted by both nuclei. This is called a covalent bond. A third type of chemical bond occurs when several atoms share all of their outermost electrons, the valence shell. In essence, the atoms build a network held together by electrostatic forces. These are called metallic bonds.
There are 92 types of naturally occurring elements. Through chemical bonds, atoms in different elements can join together to form new substances, called composites. The characteristics of each composite are determined by the type of chemical bond holding its atoms together. Ionic composites are relatively weak and dissolve in water. One example of an ionic composite is sodium chloride, or table salt. Covalent composites have a wider variety of characteristics. In nature they occur as solids, liquids or gases. Water, alcohol and DNA are among the most common covalent composites. Lastly, metals are resistant, malleable and conduct electric energy.
Sometimes, however, in order to reach a more stable electric configuration, an atom captures one or more of another atom’s electrons. As a result the number of its electrons is superior to its protons, and the atom’s charge becomes negative.
This type of atom is known as an anion. Meanwhile, an atom that loses electrons has more protons than electrons, and its charge becomes positive. This kind of atom is called a cation. Since they have opposite charges, the anion and the cation have a strong reciprocal attraction: an ionic bond. But ionic bonds are only one of the various chemical bonds possible. Another kind of bond is formed when two atoms share one or more pairs of electrons. Each of the shared electrons is attracted by both nuclei. This is called a covalent bond. A third type of chemical bond occurs when several atoms share all of their outermost electrons, the valence shell. In essence, the atoms build a network held together by electrostatic forces. These are called metallic bonds.
There are 92 types of naturally occurring elements. Through chemical bonds, atoms in different elements can join together to form new substances, called composites. The characteristics of each composite are determined by the type of chemical bond holding its atoms together. Ionic composites are relatively weak and dissolve in water. One example of an ionic composite is sodium chloride, or table salt. Covalent composites have a wider variety of characteristics. In nature they occur as solids, liquids or gases. Water, alcohol and DNA are among the most common covalent composites. Lastly, metals are resistant, malleable and conduct electric energy.
RELATED


GULL


GIRAFFE


SQUID


DONKEY


DOG
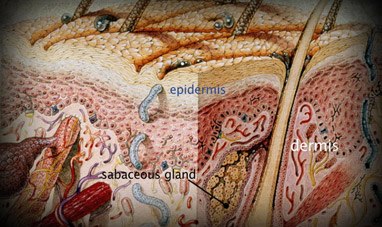

SKIN


SHEEP


GECKO


THE OCTOPUS


EOLIC


THE PRAYING MANTIS
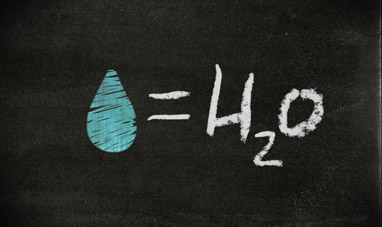

H2O

THE MOON


KINETIC, POTENTIAL AND MECHANIC ENERGIES


FLEA

COMMON COLD

ERIS


OIL


IGUANA


TWITTER


BLACK HOLES


DESERTS

THE BRAIN

THE FEMALE REPRODUCTIVE SYSTEM


COBRA

CLOUD COMPUTING


METEORS


AIDS


HYENA


GARLIC


FLAMINGO


FLY


SEA URCHIN

HYBRID VEHICLE


PENGUIN


SCORPION